Giving the hydrogen economy an acid test

A team of researchers led by the Institute of Applied Physics at the University of Tsukuba has demonstrated a method for producing acid-resistant catalysts by covering them with layers of graphene. They show that using few layers allows for greater proton penetration during a hydrogen evolution reaction, which is crucial for maximizing the efficiency when producing H2 as fuel. This work may lead to industrial-scale manufacturing of hydrogen as a completely renewable energy source for vehicles that do not contribute to climate change.
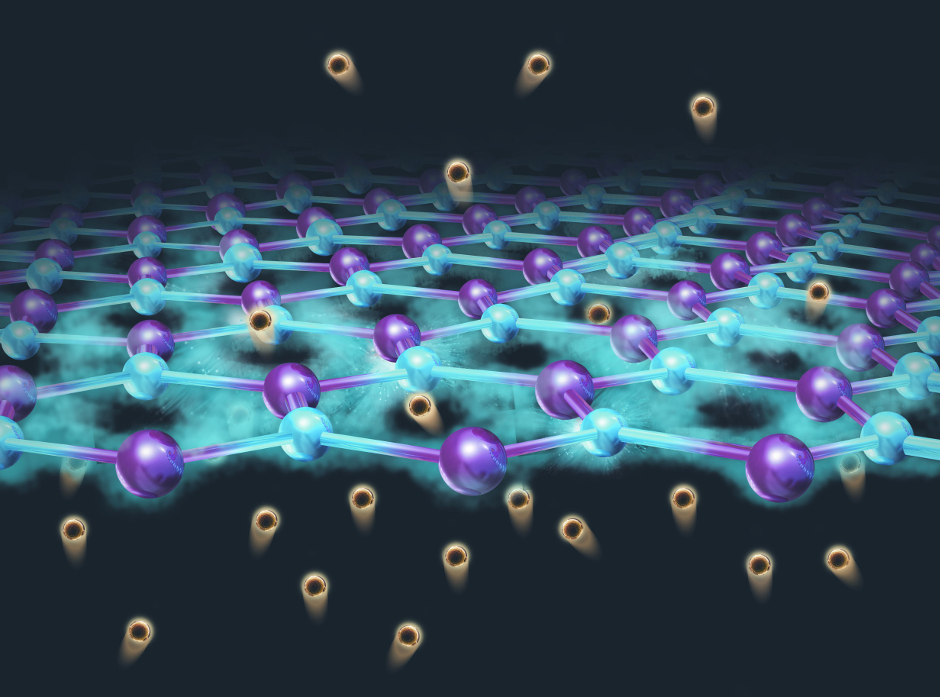
The dream of hydrogen-powered cars has excited many people as a solution for the huge amount of carbon dioxide fossil-fuel burning vehicles emit into the atmosphere daily. However, the production of hydrogen gas has been slowed by the lack of cheap catalysts required to split water efficiently. In this process, hydrogen nuclei, called protons, need to combine to form hydrogen gas, H2. Nickel and Ni-based alloy are seen as promising cheap alternatives to platinum, but these metals corrode easily when exposed to the acidic conditions of the reaction. One solution is to use graphene, a single sheet of carbon atoms arranged in a honeycomb lattice, to protect the catalyst. However, the mechanism by which the reaction takes place remained poorly understood.
Now, an international research collaboration led by the University of Tsukuba has shown that using three-five layers of graphene can efficiently prevent corrosion while still partly allowing protons to combine at the catalyst through defects in the honeycomb structure. In addition, they found that the catalytic efficiency decreased linearly as more layers of graphene were added.
"This result allowed us to conclude that protons must penetrate through the graphene layers in order to react at the surface of the metal," says Dr. Kailong Hu, senior author on the study. The alternative explanation, that electrons travel up from the metal so the protons can react at the outer surface of the graphene, was not a major reaction process supported by the experiments. Future work will focus on the optimization of the number of graphene layers to balance the corrosion resistance with catalytic activity.
"Hydrogen fuel is particularly eco-friendly because it produces zero greenhouse gasses, and still has a larger energy density than gasoline," Professor Yoshikazu Ito explains. "So we may soon be able to step on the accelerator without leaving a carbon footprint."